Background Introduction
Cardiovascular diseases mainly include coronary artery disease and other cardiovascular diseases, arrhythmia, heart failure, etc. The cure rate of the cardiovascular diseases with current treatment is still low. Cardiovascular diseases provoke threats to mankind health especially to the middle-aged and elderly with their high morbidity, disability and mortality. Methods for clear diagnosis serve as prior support including ECG, myocardial markers detection and CT in a bid to treat cardiovascular diseases.
Among them, detection of myocardial markers is a biochemical indicator reflecting heart diseases. Cardiovascular diseases develop from risk factors, cardiovascular pathological changes, cardiovascular diseases occurrence, organ system failure to death with each stage accompanied by corresponding biomarkers. The commonly used myocardial markers include three categories:
1. Markers of myocardial or vascular inflammatory reaction represented by CRP.
2. Myocardial injury markers represented by MYO, CTNT, CTNI, CK-MB, etc. are featured with high sensitivity and specificity to myocardial injury.
3. Markers of cardiac function injury, heart failure and hemodynamic disorders represented by BNP.
Recommended Diagnostic Raw Materials
[Product Information]
[Product Performance]
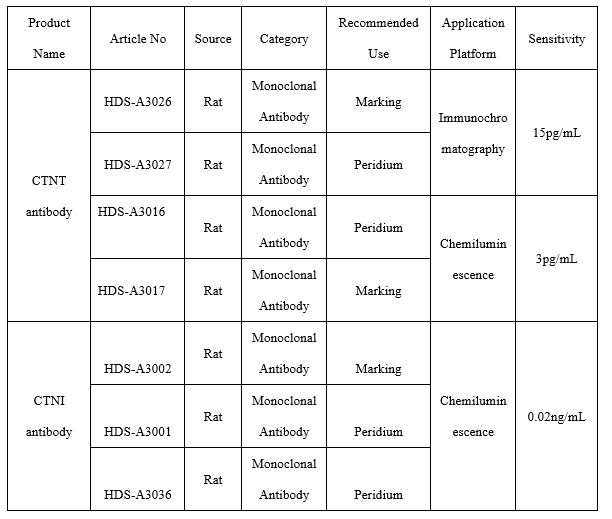
●CTNT antibody
Fluorescence Tomography Platform
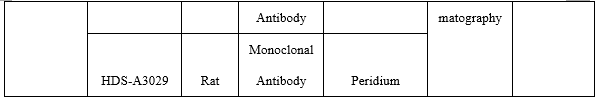
On the fluorescence chromatography platform, the CTNT paired monoclonal antibody was used to detect the serum assigned by R company renowned internationally with a sample agreement rate of R2>98%.
Chemiluminescence Platform
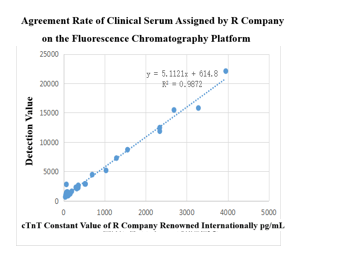
On the chemiluminescence platform, the CTNT paired monoclonal antibody was used to detect the serum assigned by R company renowned internationally with a sample agreement rate of R2>98%.
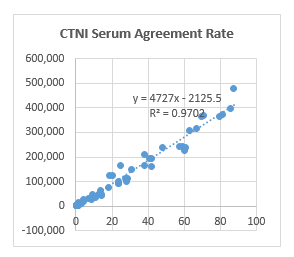
●CTNI antibody
Fluorescence Tomography Platform
On the fluorescence chromatography platform, the CTNI paired monoclonal antibody was used to detect the serum assigned by R company renowned internationally with a sample agreement rate of R2>98%.
Chemiluminescence Platform
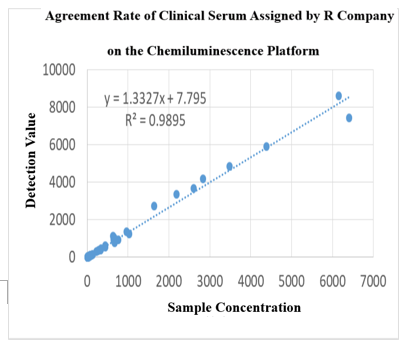
On the chemiluminescence platform, the CTNI paired monoclonal antibody was used to detect the serum assigned by R company renowned internationally with a sample agreement rate of R2>97%.
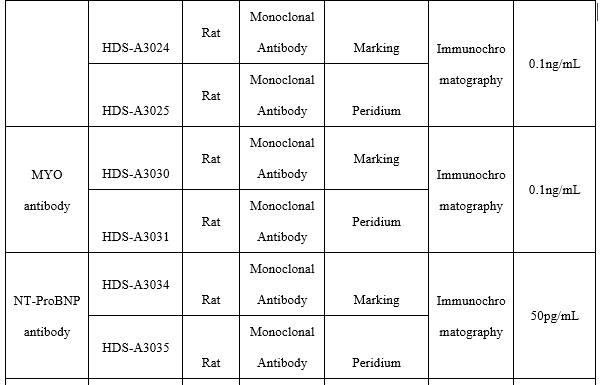
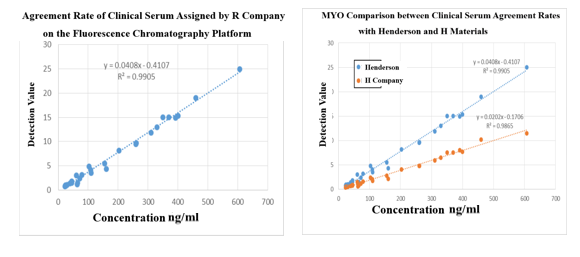
●MYOantibody
Fluorescence Tomography Platform
(1) On the fluorescence chromatography platform, the MYO paired monoclonal antibody was used to detect the serum assigned by R company renowned internationally with a sample agreement rate of R2>99%.The agreement rate of raw materials offered by Henderson is higher than that of well-known manufacturer H after the comparison of the results of serum detection with the raw materials from Henderson and H.
(2)On the fluorescence chromatography platform, the MYO paired monoclonal antibody test showed no cross reaction with diluent, 5ug/mL CTNI, 1ug/mL CK-MB, 0.1ug/mL CK-MB, 4ug/mL CTNT and 5mg/mL human hemoglobin.
Colloidal Gold Platform
(1)On the colloidal gold platform, the minimum detection limit of MYO antibody pairing was 0.29ng/mL.
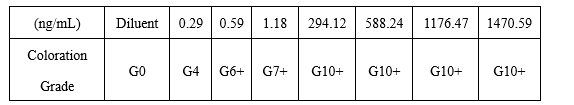
(2)On the colloidal gold platform, the MYO paired monoclonal antibody test showed no cross reaction with 2mg/mL, 10mg/mL human hemoglobin.
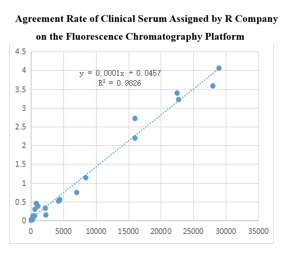
●NT-ProBNP antibody
Fluorescence Tomography Platform
(1)On the fluorescence tomography platform, the proBNP paired monoclonal antibody was used to detect the serum assigned by R company renowned internationally with a sample agreement rate of R2>98%.
(2)On the fluorescence tomography platform, the proBNP paired monoclonal antibody test showed no cross reaction with 5ug/mL CTNI, 250NG/mL MYO, 1ug/mL CK-MB.
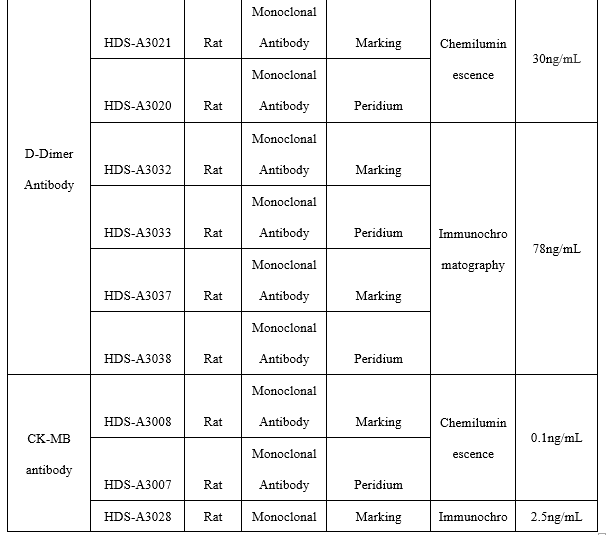
●D-Dimer antibody
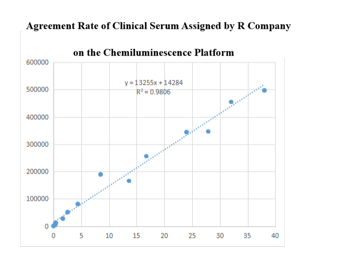
Chemiluminescence Platform
(1) On the chemiluminescence platform, the D-Dimer paired monoclonal antibody was used to detect the serum assigned by S company renowned internationally with a sample agreement rate of R2>98%.
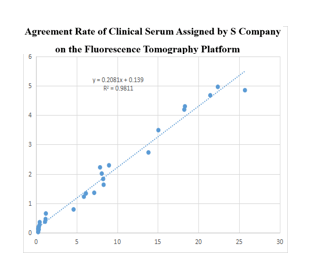
Fluorescence Tomography Platform
(1) On the fluorescence chromatography platform, the D-Dimer paired monoclonal antibody with time-resolved method was used to detect the serum assigned by S company renowned internationally with a sample agreement rate of R2>98%.
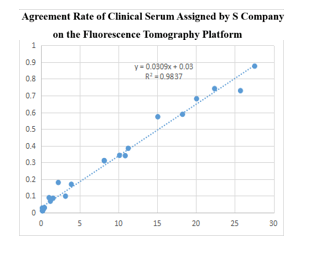
(2)On the fluorescence chromatography platform, the D-Dimer paired monoclonal antibody with direct amplification method was used to detect the serum assigned by S company renowned internationally with a sample agreement rate of R2>98%.
[Company Background]
Qingdao Henderson Biotechnology Co., Ltd, established in 2016, serves as a professional supplier of core raw materials for in vitro diagnostic reagents and is committed to providing high-quality raw materials for global in vitro diagnostic reagent production enterprises, scientific research institutions and other fields.
The company provides the following staple products: antigens, antibodies, enzymes, etc., widely used in the detection of drug, respiratory infectious diseases (A, B, COVID-19 antigen, COVID-19 antibody, COVID-19 neutralizing antibody, syncytial virus, etc.), other infectious diseases (hepatitis, AIDS, tuberculosis, etc.), markers for tumor, inflammation, myocardial markers, animal diseases detection (dog, cat diseases) and other fields.
The company, after years of development, has established several specialized laboratories and SPF experimental animal bases, a comprehensive and systematic R&D verification platform and formed a professional technical service team to provide personalized customized services and one-stop solutions.
Qingdao Henderson Biotechnology Co., Ltd is willing to build a renowned product brand for all customers with high-quality products and professional services to keep a foothold in the market with its sound competitiveness.

Limited space only allows the introduction of major products. You are welcomed to contact Qingdao Henderson Biotechnology Co., Ltd and acquire trial samples if you need more information.
Qingdao Henderson Biotechnology Co., Ltd.
Official Website: /
Tel: 0532-84670782
E-mail: info@qdhenderson.com